

| 1. |  |
2. |  |
| Experimental Setup | Ammonium chloride gas |
| Photo 1: | Ammonia Production: Sodium hydroxide pellets are placed in a 250 ml double-necked flask.
A saturated ammonium chloride solution is carefully dripped from a dropping funnel that has been pressure equalized.
Ammonia is formed according to:
NH4Cl + NaOH > NH3 + NaCl + H2O
|
| Photo 2: | Proof of Ammonium Chloride: The ammonia gas that forms is guided into a glass beaker filled with hydrochloric acid.
A white ammonium chloride fog (salmiac) is created according to: NH3 + HCl > NH4Cl
This experimental setup presents a common procedure for producing ammonia in the lab. However, for industrial purposes ammonia is manufactured from raw elements according to the Haber-Bosch Procedure:3 H2 + N2 > 2 NH3
During this exothermic (reaction enthalpy: - 92.28 kJ/mol) reaction, the volume decreases, and with decreasing temperature and increasing pressure the balance shifts to the right side, i.e. the optimal NH3 replacement is
theoretically realized by a low temperature and high pressure. However, at room temperature the speed of replacement is unmeasurably small.
Catalyzers currently in use first have an effect when temperatures exceed 400°C. However, at this temperature and atmospheric pressure the ammonia yield lies in the region of only 1 percent, which is not economically acceptable.
For this reason, one generally works with a higher pressure, in the neighborhood of 150-250. 105 Pa. In this manner, a yield in the neighborhood of 15 percent is achieved.
|
| Exercise 4.4.22.1: Solution: |
Using Hess’ Theorem, calculate the energy of formation for ammonium chloride
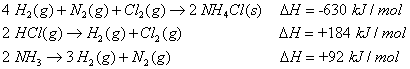 |
| Exercise 4.4.22.2: Solution: |
In a stoichiometric mixture of N2 and H2 at equilibrium at 200 bar and 470 °C, 20 percent of the volume is ammonia. Calculate the equilibrium constant for these conditions. |