Solutions to the Exercises
Solution to Exercise 2.7.1.1
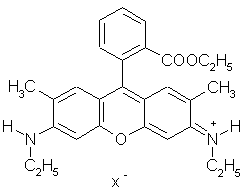
Solution to Exercise 2.7.1.2
The factor X has the units Js (Joule×second).
The constant in question is Plank's constant (6.626×10-34Js).
Solution to Exercise 2.7.1.3
The Rydberg Formula yields a frequency of n=6.17×1014.
Using l = c / n
we get the transitional wavelength 486.1 nm, corresponding to the blue
line in the spectrum of hydrogen.
Solution to Exercise 4.4.1.1
97.8 °C = 370.95 Kelvin = 208.04 °Fahrenheit
Solution to Exercise 4.4.1.2
Compared to the volume of the container, the volume of the liquid phase
can be ignored. The ideal gas law (pV = nRT) gives a post-reaction
pressure of 530 mbar.
Solution to Exercise 4.4.13.1
41.85 g aluminum are necessary.
Solution to Exercise 4.4.13.2
A reaction using 2 mol aluminum releases 852 kJ. To raise the temperature
of 200 g of water from 20 °C to 80 °C, one needs a heat amount
of 200 g × 60 K ×
4.184 J / (g × K) = 50.21 kJ. The required
amount of aluminum comes to 3.18 g.
Solution to Exercise
4.4.22.1
The formation of 1 mol NH4Cl produces 177 kJ heat.
Solution to Exercise 4.4.22.2
Given that only the gas forming reaction partners are present, the
mass action law is formulated using partial pressures rather than concentrations:
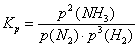
The equilibrium constant Kp has the value 6.25×10-4
bar-2.
Solution to Exercise 5.6.1
Radioactive decay is the only reaction in which reaction speed is independent
of temperature.
Solution to Exercise 5.7.1
When 50% of the original hydrogen peroxide has decayed, then c(A) =
1/2×c0(A). This yields: t1/2
= ln 2 / k
Solution to Exercise 8.5.3.1
The gas container contains 0.164 kg nitrogen.
Solution to Exercise 8.5.3.2
The mean speed of N2 molecules at 0 °C is 493.3 m/s.
Solution to Exercise OC 1.2.1
The light must have a wavelength of at least 494 nm or less. (As a
comparison, blue light has a wavelength of roughly 470 nm.)
Back to the index

