



| 1. |  |
2. |  |
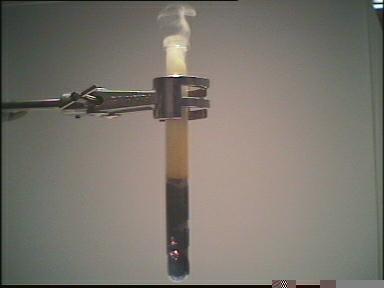
| Elements used: sulfur and iron filings | Heating the mixture | ||
| 3. |  |
4. |  |
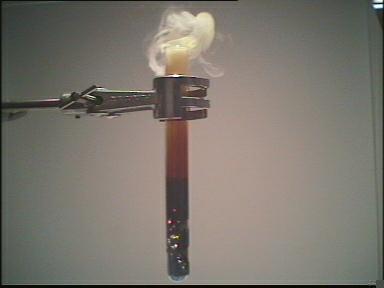
| The reaction begins | Rising sulfur vapor | ||
| 5. |  |
6. |  |
| The sulfur vapor ignites | The reaction product is not magnetic |
| Photo 1: | 5 g iron filings and 5 g sulfur are mixed in a test tube. |
| Photo 2: | The mixture is heated using a Bunsen burner. |
| Photo 3: | The reaction takes place quickly, as soon as the sulfur has melted.
Iron reacts with sulfur in a simple, exothermic reaction to produce iron
sulfide:
Fe + S ® FeS
|
| Photo 4: | The heat produced vaporizes the sulfur. |
| Photo 5: | Eventually the sulfur vapor ignites and burns with a light blue flame. |
| Photo 6: | The residue that forms is not magnetic.
Iron sulfide reacts with hydrochloric acid to produce hydrogen sulfide and iron chloride: FeS + 2 HCl® FeCl2 + H2S |