

| 1. |  |
2. |  |
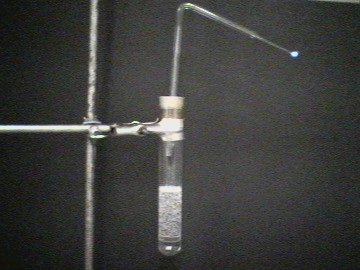
| Experimenal setup | Rising carbon monoxide gas bubbles | ||
| 3. |  |
4. |  |
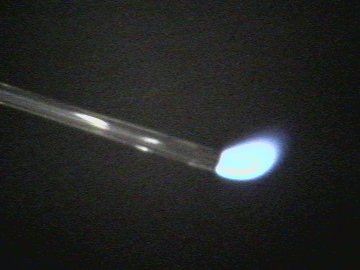
| Carbon monoxide burns with a light blue flame | Close-up of flame |
| Photo 1: | Experimental setup: 20 ml concentrated sulfuric acid and 40 ml formic acid are placed in a test tube. It is plugged with a perforated rubber stopper containing a bent glass tube. |
| Photo 2: | The concentrated sulfuric acid dehydrates the formic acid. Carbon monoxide is produced:
(H2SO4)
HCOOH
>
CO + H2O |
| Photo 3, 4: | Carbon monoxide is combustible and burns in air with a bluish flame, changing to carbon dioxide. This is a standard procedure for producing carbon monoxide in the lab. |
| Safety Precautions: | Carbon monoxide is a highly poisonous gas. Its affinity to hemoglobin in the blood is approx. 300 times larger than of oxygen. For this reason, it replaces oxygen in the O2-hemoglobin, and disrupts its function as an oxygen carrier. This causes cell respiration to stop, leading to death. The particular danger of a CO poisoning lies in the fact that a poisoned person cannot simply be saved by transporting them to a CO-free, oxygen-rich environment, since the hemoglobin remains blocked. First artificial respiration with overpressurized pure oxygen must be performed to return the hemoglobin to its original function and with it cell respiration. |