
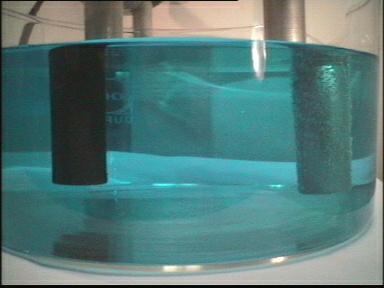


| 1. |  |
2. |  |
| Experimental setup | The reaction begins | ||
| 3. |  |
4. |  |
| Formation of chlorine at the anode | Reduced copper at the cathode |
| Photo 1: | Experimental setup: A copper(II) chloride solution is placed in a glass beaker. Two high-grade steel electrodes connected to a direct current source are placed in the solution. |
| Photo 2: | The solution dissociates at the electrodes when the current is turned on. |
| Photo 3: | Chlorine ions are oxidized at the anode according to:
2 Cl- ®
Cl2 + 2 e-
|
| Photo 4: | Copper(II) ions are reduced to elemental copper at the cathode:
Cu2+ + 2 e-®
Cu |